mRNA Technology Transfer Programme Building Sustainable Vaccine Manufacturing Ecosystems in LMICs
mRNA Technology Transfer Programme Building Sustainable Vaccine Manufacturing Ecosystems in LMICs
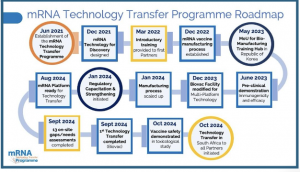
CAPE TOWN, WESTERN CAPE , SOUTH AFRICA, November 21, 2024 /EINPresswire.com/ -- The groundbreaking mRNA Technology Transfer Programme, launched in 2021, has gone from zero mRNA manufacturing capabilities in low- and middle-income countries (LMICs) to certain establishment by 2030 of 11 state-of-the-art good manufacturing practices (GMP) certified mRNA manufacturing facilities across 10 countries in the Global South.
This was shared with delegates to the mRNA Technology Transfer Programme meeting held in Cape Town from November 19 to 21, 2024.
"After successfully developing a COVID-19 vaccine as proof of concept, the Programme is now expanding to address many other diseases. It’s poised to establish a sustainable mRNA vaccine production capacity that will benefit millions across the Global South, truly redefining health equity can on a global scale," Charles Gore, Executive Director of the Medicines Patent Pool told delegates.
With all manufacturers in the Programme working on research and development (R&D) across various diseases, the network is designed to meet the Global South's R&D and mRNA vaccine needs. It stands ready to respond to any future pandemic to secure mRNA vaccine access across continents.
Established by the World Health Organization (WHO) and the Medicines Patent Pool (MPP), the Programme works with the South African Consortium, Afrigen, Biovac; the South African Medical Research Council (SAMRC); Department of Science and Innovation; and partners in Kenya, Brazil, Indonesia, India, Egypt, Nigeria, Ukraine, Bangladesh, Senegal, Tunisia, Serbia, Pakistan, Vietnam, and Argentina.
It’s supported by South Africa, France, Belgium, Canada, the European Union, Germany, Norway, and the ELMA Foundation and has propelled LMICs to the forefront of pandemic preparedness. It represents an unprecedented global effort to ensure equitable health solutions, enabling LMICs to respond rapidly and independently to global health crises.
Despite remarkable progress, an estimated US$200 million is needed to advance all manufacturers to GMP standards and continue to strengthen the R&D pipeline in support of at least 12 mRNA products currently in development. Encouragingly, Programme success has already attracted substantial catalytic co-investments. For example, for every dollar contributed by the Programme in the AFRO region, an estimated US$17 has been invested by regional stakeholders and other public health organisations.
Prof. Petro Terblanche, CEO of Afrigen, said: "The mRNA Programme has not only achieved initial goals but exceeded them in every way. Afrigen's work with global partners has shown that LMICs can lead in R&D and manufacturing, transforming healthcare outcomes from diseases that affect the Global South. This Programme demonstrates the power of partnerships and global collaborations."
Dr Martin Friede, Coordinator at WHO, emphasised, "We are committed to securing the necessary support to see these efforts through so that LMICs have the scientific and material resources to maintain this unprecedented level of pandemic preparedness."
About the mRNA Technology Transfer Programme
The mRNA Technology Transfer Programme was launched in 2021 by the World Health Organization (WHO) and the Medicines Patent Pool (MPP) to address the need for equitable access to mRNA vaccine technology in low- and middle-income countries (LMICs). This initiative aims to build mRNA manufacturing capacity in LMICs, empowering them to produce mRNA vaccines locally and ensuring rapid response capabilities in the event of future pandemics. By fostering collaboration among a global network of partners, the Programme advances research and development, supports the establishment of Good Manufacturing Practices (GMP)-certified facilities, and enables LMICs to achieve self-sustaining vaccine production.
About WHO
The World Health Organization (WHO) is a specialised agency of the United Nations dedicated to promoting global health and well-being. Founded in 1948, WHO leads efforts to combat infectious diseases, address health emergencies, and promote universal health coverage worldwide. Through its partnerships, policymaking, and capacity-building initiatives, WHO plays a crucial role in coordinating global health responses and supporting countries in achieving equitable access to healthcare. In the context of the mRNA Technology Transfer Programme, the WHO provides strategic guidance and technical support to facilitate vaccine access and preparedness in LMICs.
About Afrigen
Afrigen Biologics and Vaccines, headquartered in Cape Town, South Africa, is a biotechnology company dedicated to advancing vaccine and biological production in Africa. As a key partner in the mRNA Technology Transfer Programme, Afrigen has spearheaded efforts to develop and manufacture Africa's first mRNA COVID-19 vaccine, AfriVac 2021. With a mission to foster health innovation and capacity-building in the Global South, Afrigen leads in R&D, technology transfer, and training, playing an essential role in building a self-sufficient, resilient biomanufacturing network for Africa.
About the Medicines Patent Pool (MPP)
MPP is a United Nations-backed public health organisation focused on improving access to life-saving medicines in LMICs. Established in 2010 by Unitaid, MPP works with partners to license essential medicines and health technologies, fostering innovation and affordability in the areas of HIV, hepatitis C, tuberculosis, non-communicable diseases and COVID-19. MPP's role in the mRNA Technology Transfer Programme includes coordinating with manufacturers, ensuring sustainable R&D practices, and facilitating technology sharing to accelerate mRNA vaccine production across LMICs.
Edwin Reichel
Flow Communications
email us here
Visit us on social media:
X
LinkedIn
YouTube
Distribution channels: Business & Economy, Chemical Industry, Healthcare & Pharmaceuticals Industry, International Organizations, Technology
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release